Product Consultation
Your email address will not be published. Required fields are marked *


The Brazilian partner of Hangzhou Zhengda Medical Equipment Co., Ltd. has officially obtained the Brazilian medical device registration certificate for the company’s touchscreen automatic tourniquet system, marking an important milestone in entering the Latin American market and confirming compliance with the strict regulatory requirements of the Brazilian Health Regulatory Agency (ANVISA).

ANVISA: Brazil’s Strict Health Regulatory Authority
The Agência Nacional de Vigilância Sanitária (ANVISA), operating under Brazil’s Ministry of Health, is the national authority responsible for regulating medical devices, pharmaceuticals, and healthcare products. As part of the Brazilian National Health System (SUS), it plays a critical role in ensuring the quality, safety, and efficacy of medical technologies across the country.
Visit ANVISA Official Website:https://www.gov.br/anvisa/en
The Brazilian Medical Device Registration Process
The ANVISA approval process is one of the most demanding worldwide and requires medical device manufacturers to complete a series of steps including the review of clinical data to demonstrate patient safety and treatment effectiveness, detailed product performance testing to confirm compliance with technical standards, and quality management system (QMS) audits at the manufacturing facilities. In addition, comprehensive technical documentation and regulatory evidence must be submitted to prove that the device meets both international and Brazilian standards, ensuring that only products of the highest reliability can enter the Brazilian healthcare market.

Challenges: Time, Cost, and Compliance
The path to Brazilian registration is not only complex but also requires significant time and financial resources. Review periods frequently extend beyond one year, while registration fees and ongoing compliance costs remain relatively high. These challenges demand sustained investment in technology R&D, robust quality control, and strong regulatory expertise, meaning that only companies with a solid technical foundation and long-term commitment are able to successfully navigate this process. The approval of the touch screen automatic tourniquet system demonstrates Hangzhou Zhengda Medical’s ability to meet international standards and highlights its resilience in managing regulatory and operational challenges.
Global Outlook: Strengthening International Collaboration
With this successful registration in Brazil, Hangzhou Zhengda Medical reaffirms its dedication to expanding international cooperation. The company aims to build long-term partnerships in areas such as technology research and development, market expansion, and regulatory compliance, while continuing to contribute to the improvement of global healthcare services. By working hand in hand with international medical peers, the company seeks to achieve mutual benefit, promote innovation, and create new opportunities for the medical device industry worldwide.

Automatic tourniquet system safeguards clinical medical care — a special training session at a Shandong hospital concluded successfully
Aug 15,2025
Hangzhou Zhengda Medical engineers went to a hospital in Guangzhou to conduct special training on lower limb joint rehabilitation device CPM-E
Sep 04,2025Your email address will not be published. Required fields are marked *

Spine Operating Support

Medical Ultrasonic Atomization System

Expectoration Therapy Instrument Series
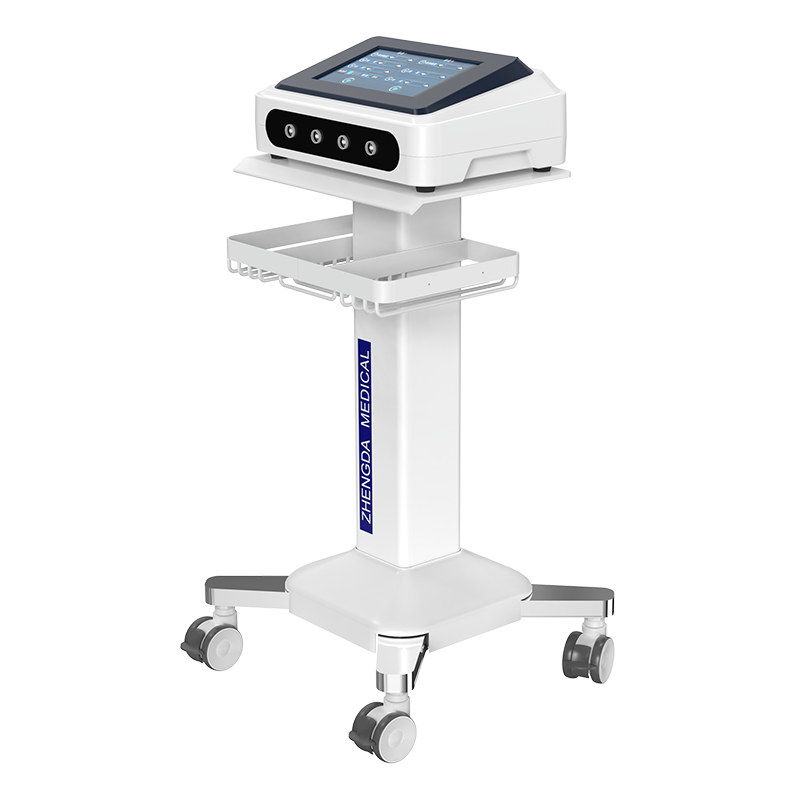
Magnetic therapy products
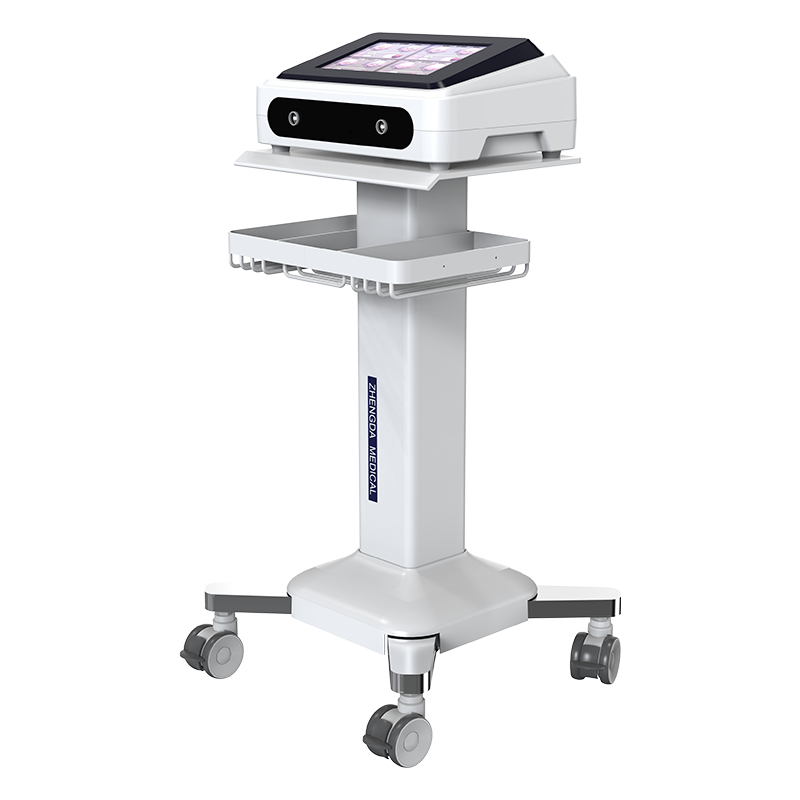
Electrotherapy series products
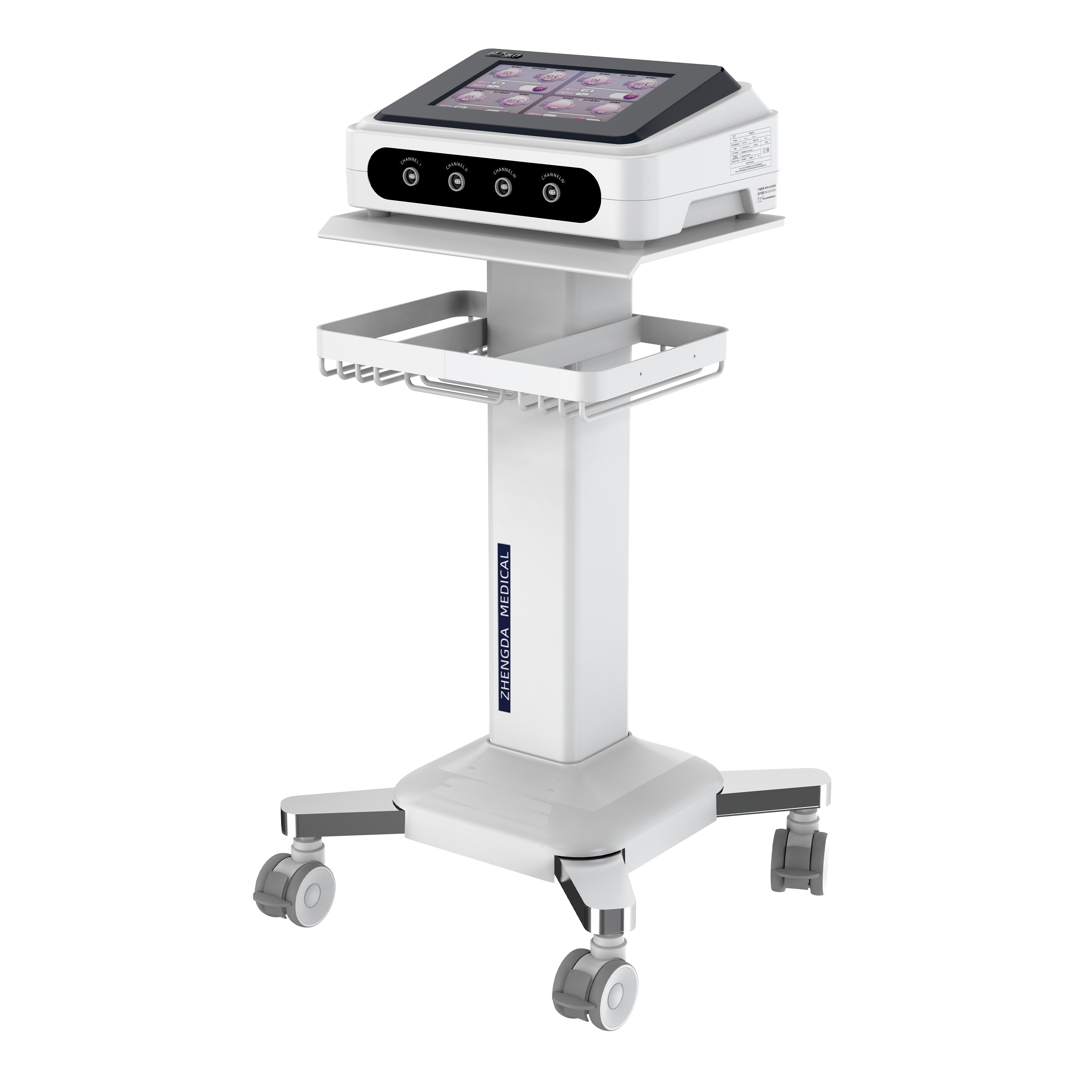
Electrotherapy series products

Cervical and Lumbar Traction System
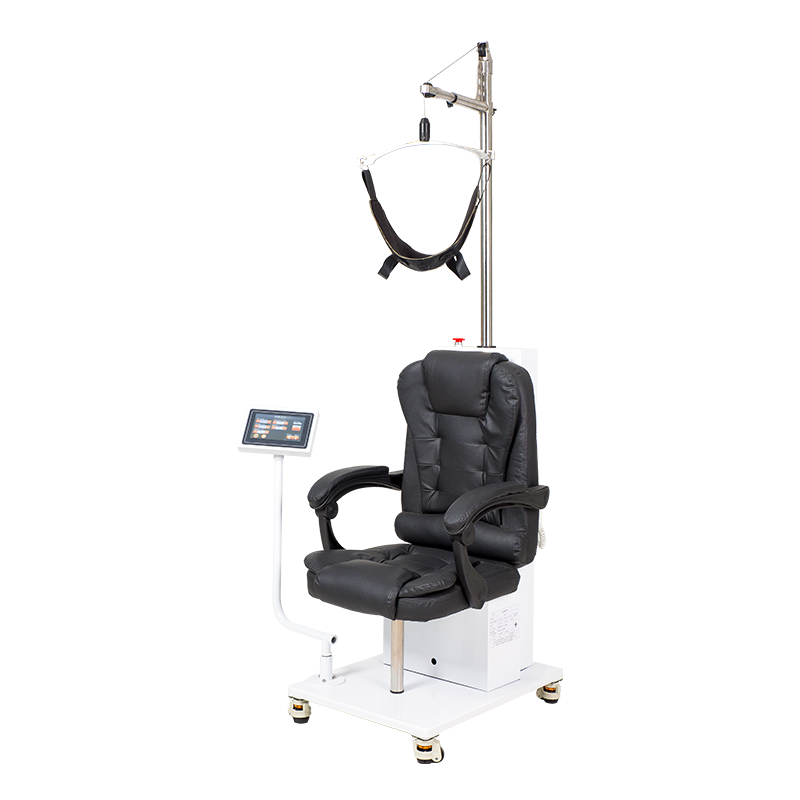
Cervical and Lumbar Traction System

Pneumatic Finger Trainer

Massage System

Orthopaedic power tools

Compression Therapy System
COPYRIGHT © Hangzhou Zhengda Medical Equipment Co.,Ltd ALL RIGHTS RESERVED.
Custom medical equipment Manufacturers
The information provided on this website is intended for use only in countries and jurisdictions outside of the People's Republic of China.