Product Consultation
Your email address will not be published. Required fields are marked *


In today’s increasingly competitive global medical market, securing overseas certification for medical devices is often fraught with challenges. Recently, Hangzhou Zhengda Medical Equipment Co., Ltd.(hereinafter referred to as Hangzhou Zhengda Medical) achieved a significant milestone: its self-developed Electric Pneumatic Tourniquet System successfully obtained Thailand Medical Device Registration. This accomplishment not only marks a pivotal step in the company’s international expansion but also underscores Hangzhou Zhengda Medical’s expertise in the medical device industry.
Ⅰ. Product Excellence: The Foundation for Certification
The Automatic Tourniquet System certified by Thailand’s regulatory authority is one of Hangzhou Zhengda Medical’s flagship products. Designed to meet clinical demands, the device features:
Precise pressure control for optimal safety.
Reliable hemostatic performance to enhance surgical outcomes.
User-friendly operation for streamlined workflows.
Widely recognized by medical institutions globally, this device provides critical support for intraoperative hemostasis.

Ⅱ. Overcoming Three Key Certification Challenges
Thailand’s medical device registration process is rigorous, requiring comprehensive reviews of technical documentation, clinical data, and quality management systems. During the initial phase, Hangzhou Zhengda Medical’s team navigated major hurdles:
Regulatory Differences: Thailand FDA (TFDA) requirements diverge from those in Western markets, necessitating adjustments to technical files for local compliance.
Language Barriers: Submission documents required precise Thai translations, with technical terminology posing a particular challenge.
Prolonged Approval Timelines: Thailand’s stringent review process meant even minor discrepancies could delay certification.
Through meticulous preparation, cross-border collaboration, and unwavering commitment to quality, Hangzhou Zhengda Medical’s successfully addressed these obstacles, securing the registration.

Ⅲ. Strategic Impact of the Certification
This certification authorizes the legal sale and use of Hangzhou Zhengda Medical’s Electric Pneumatic Tourniquet System in Thailand, empowering local healthcare providers with advanced technology. Looking ahead, Hangzhou Zhengda Medical remains committed to: Delivering customer-centric solutions.Accelerating global market access for high-quality medical devices.
Stay tuned as we bridge innovation with international standards, one breakthrough at a time!

Hangzhou Zhengda Medical's Lower Limb Continuous Passive Motion & Automatic Tourniquet System Secure Malaysia MDA Certification
Jul 14,2025
Good news | Hangzhou Zhengda Medical Equipment Co., Ltd. won the title of "Zhejiang Province Specialized, Special and New Small and Medium Enterprises"
Jul 14,2025Your email address will not be published. Required fields are marked *

Spine Operating Support

Medical Ultrasonic Atomization System

Expectoration Therapy Instrument Series
Magnetic therapy products
Electrotherapy series products
Electrotherapy series products

Cervical and Lumbar Traction System
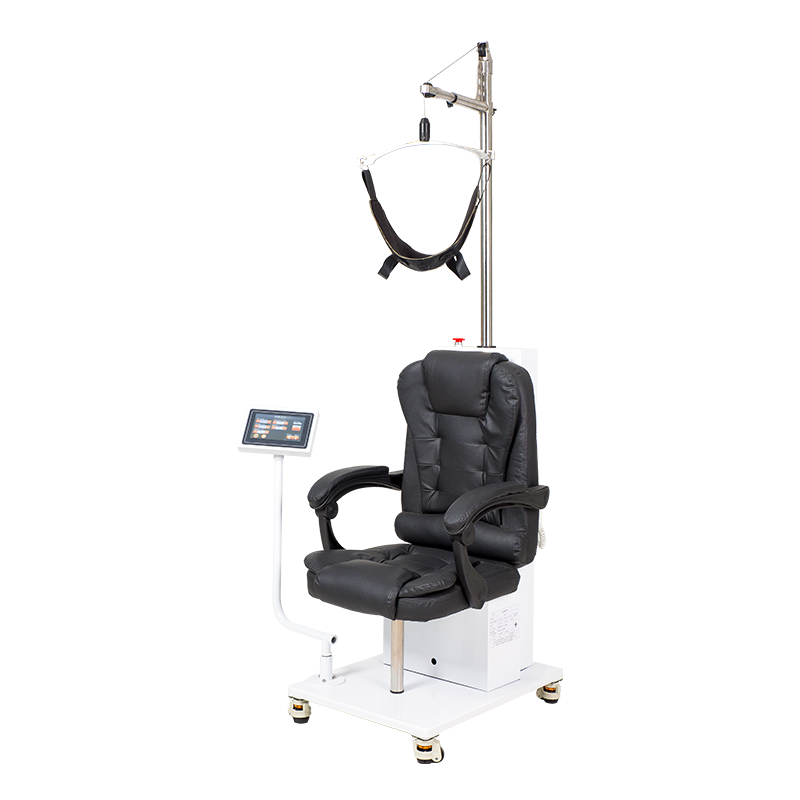
Cervical and Lumbar Traction System

Pneumatic Finger Trainer

Massage System

Orthopaedic power tools

Compression Therapy System
COPYRIGHT © Hangzhou Zhengda Medical Equipment Co.,Ltd ALL RIGHTS RESERVED.
Custom medical equipment Manufacturers
The information provided on this website is intended for use only in countries and jurisdictions outside of the People's Republic of China.